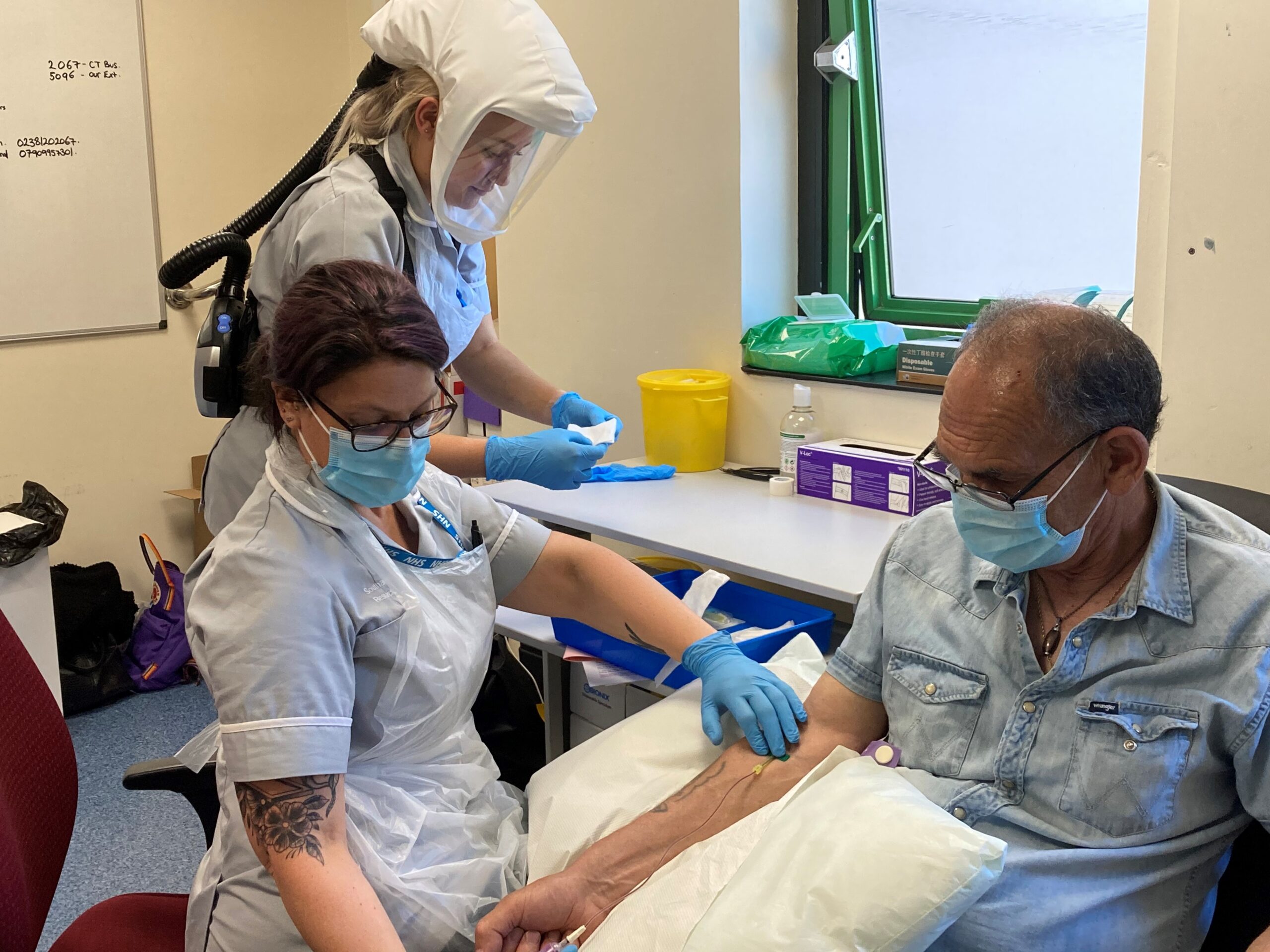
The Wessex Secure Data Environment (SDE) is playing a key role in the ‘iDx Lung’ study, a collaborative research project spearheaded by the Southampton Clinical Trials Unit, based at the University of Southampton, and the iDx Lung Consortium which recruited 7000 participants. This exciting study seeks to revolutionise early lung cancer detection through comprehensive data analysis.
Using the Wessex SDE’s sophisticated data handling capabilities, the study combines data from analysing blood and nasal swab tests, and detailed patient background information. Researchers look at this data for clues to help find lung cancer at an earlier stage, potentially increasing survival rates and improving care.
The study has already completed its first phase, which did not use the Wessex SDE. In its next phase, iDx Lung will expand to further trial sites across the UK, recruiting a further cohort of participants from across the country.
To be able to find patterns and make discoveries from all this data researchers need to combine it. More people and more places make this a really complex job. Without the support of the Wessex SDE this would be a huge challenge. With the SDE it can be done quickly and securely, with the NHS in full control of how patient health data is used.
The next phase of the study is now in development. For more information or to learn how to get involved, please contact the Southampton Clinical Trials Unit (here).