On Monday 7 April, the Prime Minister announced that the Government and the Wellcome Trust will invest up to £600 million to create a new Health Data Research Service. This groundbreaking initiative will deliver significant health benefits to the UK public and patients.
The Health Data Research Service (HDRS) will transform access to NHS data by providing a single UK-wide access point to national-scale datasets. It will provide a national health data research service, building on work across the Data for Research and Development Programme, including the NHS Research SDE Network.
Currently health data is held in a lot of different places, managed through different access processes. HDRS will streamline and simplify those processes through a “single front door” system where researchers make standardised requests to access data, making the system simpler and more efficient to accelerate the speed at which new treatments and services start to benefit patients.
The SDE Network is already helping to converge more than 7,000 existing access points. Chris Kipps, Wessex SDE Lead and Professor of Clinical Neurology and Dementia at University Hospital Southampton said:
“Working collaboratively with the SDE Network and the public, we have been establishing the vital groundwork to successfully deliver national ambitions that will benefit our local communities from health research and contribute to UK life sciences. We hugely welcome this announcement which will build on the work that the Wessex SDE is doing to improve research access to NHS data and thus accelerate key projects, such as our Pre-Hospital Research and Audit Network (PRANA), our Dementia Driver project and multiple cancer projects.”
The Wessex SDE will continue to work with patients, the public, healthcare professionals and our external stakeholders to design and develop the service and will ensure that public trust is at the heart of our work. NHS England and the Department of Health and Social Care are running a major national engagement programme on data—the first set of findings were published, collected from over 4000 people across England, in March 2025 here. We will continue engaging and involving the public and patients throughout the development of this service.
We anticipate the NHSE team and Network to work together at pace over the next few months to co-design what this looks like in practice.
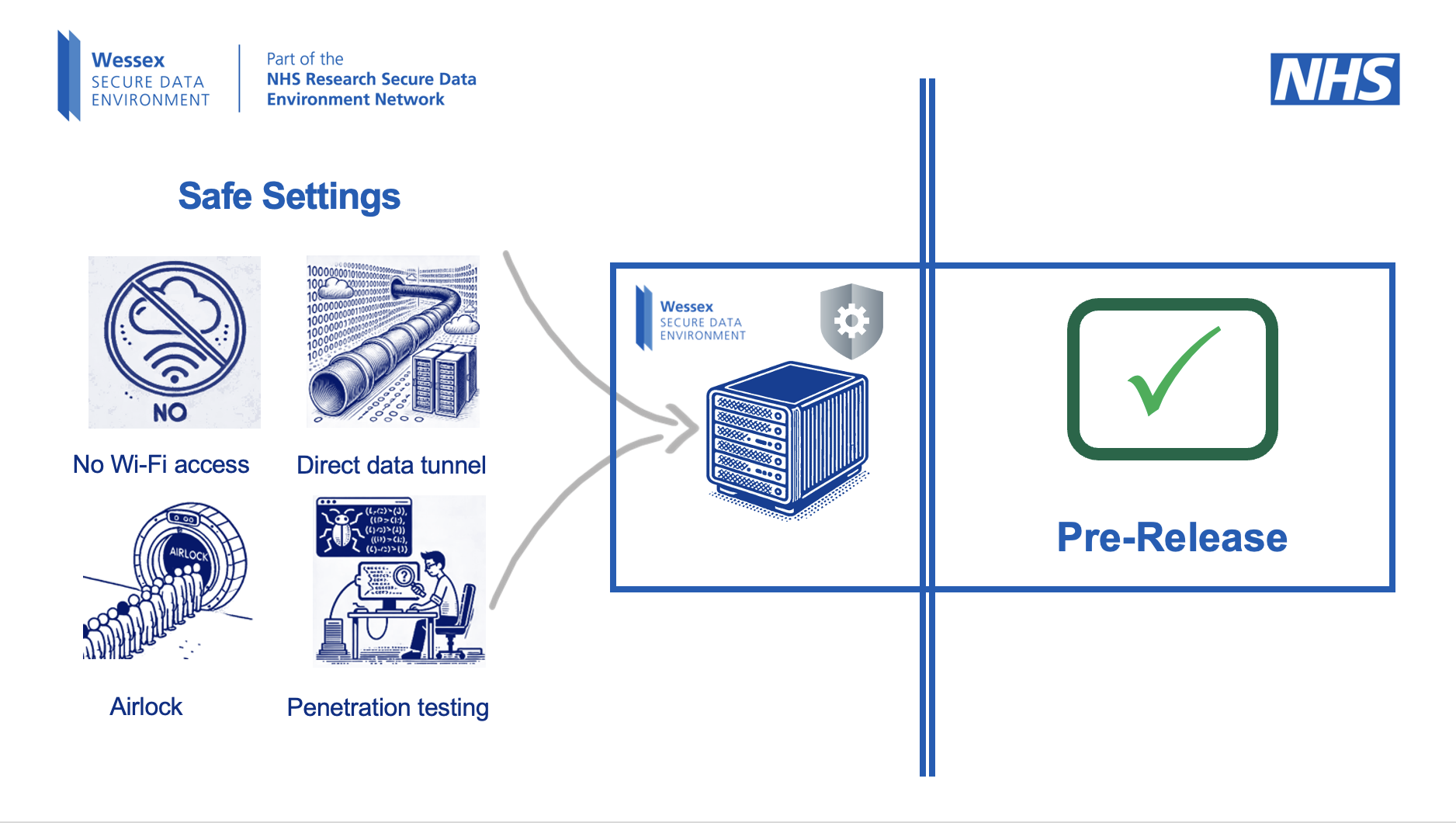
Patient confidentiality will continue to be held to a gold standard with these changes – with robust security measures being in place, like anonymity and virtual locked rooms, to ensure no one’s health data is compromised.
Professor James Batchelor, Wessex SDE Technical Lead and Fellow of Clinical Informatics and Healthcare Innovation at the University of Southampton, said:
“Wessex is already home to EDGE, a world leading clinical management research system embedded into 90% of England’s NHS hospitals, with over 10m clinical research journeys. Combining this experience with the Wessex SDE will help us to unlock the power of NHS data while delivering the gold-standard security and privacy expected by our communities.”
Details of the service governance will be shared in due course. We remain commited to codesign our governance with the public and key stakeholders including the SDE Network as well as the charities that have been involved as part of this community.
The UK Government is partnering with Wellcome Trust as they are one of the largest, most prominent and respected philanthropic organisations in the UK and one of the largest medical research charities globally.
Wellcome’s investment will support the UK Government to deliver the service more quickly but does not tie us to a specific design or delivery mode.